Blog
-

Non-Surgical Advances in Treatment for Conductive Hearing Loss.
 16 Aug , 2018
16 Aug , 2018
Non-Surgical Advances in Treatment for Conductive Hearing Loss.
Hearing via bone conduction is a phenomenon that has been recognized and explored for millenia. In more recent centuries, a better understanding of bone conduction hearing, and its use in differential diagnosis and treatment of certain types of hearing loss has emerged.
In 1551, Girolamo Cardano, an Italian physician, philosopher, and mathematician, wrote about how sound may be transmitted to the ear by means of a rod or the shaft of a spear held between one’s teeth.1 In 1603, Hieronymus Capivacci catapulted bone conduction hearing into clinical significance by describing what happened when he connected the teeth of a patient to the strings of a sitar with a 2-foot-long iron rod. He noted that if the patient was able to hear sound from the instrument, the site of lesion was the tympanic membrane, whereas if the patient did not hear anything, the auditory nerve was the site of lesion.2
We recognize his finding as an early example of differential diagnosis between conductive and sensorineural hearing loss. Through the centuries that have followed, advancements in diagnostics and treatment strategies have improved outcomes for individuals struggling with conductive hearing loss.
Beyond the realm of conductive hearing loss, advancements in hearing technology over recent decades have resulted in miniaturization of hearing devices, improved performance in challenging listening environments, and enhanced cosmetic appeal. Yet, in spite of such advancements, non-surgical options for the audiologic management of conductive hearing loss have been beset by challenges ranging from uncomfortable and cosmetically unappealing wearing options, to unreliable retention on the patient’s head.
Since the announcement of FDA clearance of the new ADHEAR System by MED-EL, we have received significant interest from clinicians, users, and caregivers excited to learn about a new option that redefines the category of skin drive, non-surgical bone conduction technology.
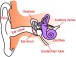
What is Conductive Hearing Loss?
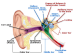
Conductive hearing loss results when sound waves cannot be effectively transmitted through the outer ear, tympanic membrane, or middle ear to the oval window. It is caused by problems with the ear canal, eardrum, or middle ear, and the smallest bones in the human body—the malleus, incus, and stapes.
Causes of conductive hearing loss include congenital absence of the ear canal or failure of the ear canal to be open at birth, and congenital absence, malformation, or dysfunction of the middle ear structures. Conductive hearing loss can also be caused by infection, tumors, middle ear fluid from infection or Eustachian tube dysfunction, a foreign body, trauma (eg, skull fracture), and otosclerosis.3
Candidacy for Bone Conduction Technologies:
Candidates for bone conduction technologies fit into three major categories: 1) those with conductive hearing loss; 2) those with mixed hearing loss; and 3) those with single-sided deafness (SSD). When considering any bone conduction system for patients, it is imperative that both a comprehensive audiological evaluation and a thorough otologic evaluation be performed. In patients with these types of hearing loss, a number of medical or otologic concerns may be present, including drainage, deformity, sudden or rapidly progressive hearing loss, dizziness, sudden or rapid unilateral hearing loss, an air-bone gap greater than 15 dB, cerumen accumulation, or a foreign body in the ear.
Most candidates with conductive hearing loss will benefit from bone conduction technology. Studies suggest that with an air-bone gap of approximately 30 dB, better results will be had with a bone conduction system than an air conduction hearing aid.4 And, of course, in cases where an air-conduction hearing aid is not possible—such as aural atresia, chronic otorrhea, or inability to tolerate an earmold—a bone conduction system is the first option regardless of the width of the air-bone gap.5
Bone conduction solutions for mixed hearing loss must not only overcome the conductive component, but also compensate for the sensorineural hearing loss. Additional power is required and examples include power and super-power options.6
With SSD, the bone conduction system is utlized to reroute sound from the deaf side to the contralateral normal-hearing ear. While this does not restore binaural hearing, it may assist in overcoming the head-shadow effect. The principal advantage over an air-conduction contralateral routing of sound (AC-CROS) system is the fact that the patient does not need to wear a second hearing device on the normal-hearing ear.
Available Treatment Options for Conductive Hearing Loss:
Medical or surgical management of the conductive hearing loss. The focus of this article is on the technologies that may be employed in the management of conductive hearing los. However, it should be noted that medical or surgical intervention may well resolve a conductive hearing loss, hence the importance of multidisciplinary care.
Hearing aids. Air-conduction hearing aids may suffice for some patients with mild conductive hearing loss and a typical anatomy. For cases where an air-conduction hearing aid is not the optimal solution, we must look at our surgical and non-surgical options in bone conduction technology.
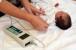
“Softband” and “hardband” devices. Non-surgical bone conduction systems are suitable for individuals who are unwilling to undergo surgery, who do not meet minimum age requirements for surgery, who may be faced with other surgeries that need to be prioritized (for example in the context of a craniofacial condition), or who have a conductive hearing loss of uncertain duration that may ultimately resolve.
A bone conduction audio processor coupled to the head with a softband has been the most common non-surgical option available over the past 15 years or so. The softband has largely replaced the hardband due to relatively improved comfort and cosmetics; however, the softband presents a number of challenges that can lead to less than ideal compliance with a clinician’s recommendations. Complaints include discomfort due to pressure when worn, concerns over cosmetic appeal, and unreliable placement on the head. Discomfort often results in limited daily wear time. Indeed, users are advised to ease into daily softband usage, wearing it for no more than 10-15 minutes at a time. Bone conduction audio processors attached to a softband are also high-profile and visible, a challenge whether the recipient is male or female, infant, toddler, child, or adult. Many adults with conductive hearing loss will refuse to consider a softband system, even for a short trial period. Placement and retention of softband systems is a known variable in the real world. The softband may slip on the head, or end up being moved around throughout the day to relieve pressure and discomfort.
Achieving compliance with the recommendations of the audiologist is challenging for parents, caregivers, and users. Reduced compliance is a particular concern for children when access to hearing is critical for language acquisition, cognitive development, and academic performance. The daily frustrations encountered may result in pressure to opt for a bone conduction implant to leave behind the challenges of softband bone conduction systems.
Bone conduction implant. Candidates may pursue bone conduction implant surgery because they are unhappy with their current device, they are old enough for the surgery, their audiologist advises them that their hearing will improve and a surgical consult confirms that they are a good candidate. However, currently available surgical options also present a number of challenges. For example, opting for an abutment-based system (percutaneous, direct-drive) means a commitment to lifelong medical treatment and maintenance of the skin site around the abutment. The complication rates for such systems are high. Other bone conduction implant systems are magnet-based, skin-drive systems (passive transcutaneous) which are also associated with skin complications and discomfort.
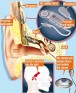
ADHEAR: Bone Conduction without Pressure
The FDA recently granted MED-EL USA clearance for the ADHEAR System, a novel non-surgical bone conduction system for people with conductive hearing loss or SSD. ADHEAR allows, for the first time, patients of all ages and stages to enjoy a system that does not apply pressure to the skin and is comfortable to wear all day.
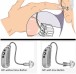
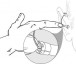
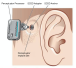
The ADHEAR System consists of a proprietary, patented, dual-interface adhesive adapter which is placed behind the ear and a low-profile audio processor which attaches to the adhesive adapter. No softband is required. The adhesive adapter is intended to be used on intact healthy skin. No skin condition that contraindicates application of an adhesive behind the ear should be present.
The ADHEAR System offers equivalent performance to conventional (standard power) softband systems. How is this possible without pressure? First, the bulky skin contact plate used in softband systems is replaced by a low-mass adhesive adapter which allows more efficient sound transfer to the bones of the skull. Second, the adhesive adapter is placed in an optimal position for stimulation of the ipsilateral cochlea. Third, the adhesive adapter is placed over hairless skin, so the system does not suffer from hair-related attenuation. Due to its adhesive positioning, AHDEAR provides consistent sound quality throughout the day. The lightweight audio processor is simply clicked on and off the adhesive adapter as desired.
The audio processor has dual microphones and digital signal processing features, such as feedback management and noise management, and is powered by a single size 13 battery which lasts up to 2 weeks. A push button allows users to switch between programs, and users can adjust the volume with the small wheel on the side of the audio processor. A cable can also be connected to the audio processor and linked to devices, such as mobile phones, computers, MP3 players, and FM and telecoil receivers.
As mentioned, the single-use adhesive adapter has dual interfaces—both optimized for the user. The first is the skin-adapter interface. The adhesive is strong enough to handle repeat attachment and removal of the audio processor without dislodging from the skin. The medical-grade, non-sensitizing adhesive adapter is worn by the patient for 3 to 7 days at a time, and can be worn while bathing or swimming.
The adapter-device interface is designed for durability of the audio processor and improved cosmetics. Wear and tear related to connecting and disconnecting the audio processor is on the adhesive adapter which has a short lifetime. The coupling plate and snap coupler arrangement is lower profile than softband arrangements, thus enhancing the cosmetic appeal of the ADHEAR system.
Availability of the ADHEAR System:
ADHEAR is available in the United States during 2018 and is priced affordably. Multiple training modalities are available to ensure clinicians are supported as they incorporate ADHEAR into their clinic offerings. Training options include online webinars, regional training events (visit www.stickclickhear.com), and consults with MED-EL audiologists.
A few highlights of the ADHEAR system make it appealing to clinicians working in a variety of settings including hospitals, ENT offices, school systems, and private practices. The first is the enhanced cosmetics and comfort of the system. Additionally, ADHEAR is easy to demonstrate so that patients can make informed decisions.
ADHEAR ships with a preset configuration suitable for many users, and optimized for an efficient demonstration process for all users. The volume control range is 16 dB, and there are four preset programs that are sequenced logically so a clinician can easily and efficiently demonstrate the system to patients of any age. While the four preset programs are designed to support typical listening environments, an optional software configuration tool allows a clinician to adjust settings, microphone modes, frequency response, number of programs, and audible indicators as appropriate for a patient’s individual needs.
While ADHEAR may be coupled to an elastic headband, its innovation lies in the appeal of the adhesive adapter wearing option. The adhesive adapters are single use, and are easier for demonstration purposes than managing a supply of demonstration softbands, or in some cases, having families pay for softbands simply to try out a system.
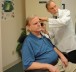
In schools, the ADHEAR system may be an appealing loaner device for children with chronic conductive hearing loss that may resolve over time, or even for children with a percutaneous abutment whose audio processor is out for repair (Figure 3). These children can wear the adhesive system, while waiting for their repaired device, without infection control concerns related to a loaner audio processor sitting on an abutment.
Great strides have been made since the discovery of bone conduction hearing and the development of technologies that capitalize on this phenomenon. A comfortable and discreet wearing experience translates to a child or adult being willing to wear a device, and longer wear times translate to more sound access. This gives adults and parents of children with conductive hearing loss leeway to move forward with bone conduction implant surgery if and when the time is right for them—no pressure with the device, no pressure with the decision.
The ADHEAR System is designed as a safe and effective option for people living with conductive hearing loss. Considering the treatment options currently available on the market for the same intended use, ADHEAR represents a welcome state-of-the-art treatment option.
Source: Amanda T. O’Donnell, AuD, The Hearing Review
Image credit: MED-ELOriginal citation for this article: O’Donnell AT. Non-surgical advances in treatment for conductive hearing loss. Hearing Review. 2018;25(9):36-38.
References
Deafness in Disguise: Timeline of Hearing Devices and Early Deaf Education. Washington University School of Medicine website. http://beckerexhibits.wustl.edu/did/timelineMudry A, Tjellström A. Historical background of bone conduction hearing devices and bone conduction hearing aids. Adv Otorhinolaryngol. 2011; 71:1-9.
Types, Causes, and Treatments of Hearing Loss. Hearing Loss Association of America (HLAA) website. https://www.hearingloss.org/…/he…/types-causes-and-treatment
De Wolf MJF, Hendrix S, Cremers CWRJ, Snik AFM. Better performance with bone-anchored hearing aid than acoustic devices in patients with severe air-bone gap. Laryngoscope. 2011;121(3)[Mar]:613-616.
Mylanus EAM, van der Pouw KCTM, Snik AFM, Cremers CWRJ. Intraindividual comparison of the bone-anchored hearing aid and air-conduction hearing aids. Arch Otolaryngol Head Neck Surg. 1998;124:271-276.
Snik AFM, Mylanus EAM, Proops DW, et al. Consensus statements on the BAHA System: Where do we stand at present? Ann Otol Rhinol Laryngol. 2005;114(12)[Dec]:2-12.