Innovative Gene Editing Method May Prevent Deafness
 29 Dec , 2017
29 Dec , 2017
Innovative Gene Editing Method May Prevent Deafness
For the first time, researchers have used an innovative, state-of-the-art genome editing technique to prevent deafness in mice. There is hope that, in the future, they will be able to use this method to stop the loss of hearing in humans.
According to data from the National Institute on Deafness and Other Communication Disorders, around two to three children in every 1,000 in the United States are born with a hearing impairment in one or both ears, and about 15 percent of adults have hearing problems.
Moreover, the Centers for Disease Control and Prevention (CDC) note that 50 to 60 percent of hearing loss cases in babies are due to genetic factors, caused by the mutation of genes that “program” hearing.
Recently, scientists have been experimenting with genome editing methods in the hope that they would be able to manipulate it so as to prevent the onset of total deafness due to genetic factors.
Researchers at the Howard Hughes Medical Institute in Chevy Chase, MD, have now used precise genome editing technology called CRISPR-Cas9 on a mouse model to remove a gene variant that can lead to total loss of hearing.
“We hope that the work will one day inform the development of a cure for certain forms of genetic deafness in people,” says David Liu, one of the researchers involved with the study.
Liu and colleagues detail the process and their findings in a paper published in the journal Nature.
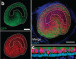
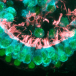
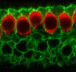
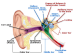
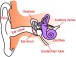
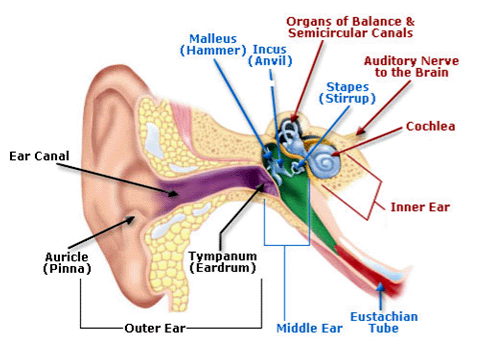
One gene that has been associated with hearing is Tmc1. Mutations in this gene have been known to cause deafness, since they trigger the loss of hair cells in the cochlea, a part of the inner ear. Cochlear hair cells play an important role in hearing; they pick up vibrations and communicate with brain cells, thereby allowing the sensation to be processed. Mutated Tmc1 causes gradual hearing loss in both humans and mice, which allowed Liu and team to use the mouse model in their research.
The scientists hypothesized that, if they could remove the mutated copy of the gene, they would be able to prevent total loss of hearing in the animals.
Working with young mice, the researchers used CRISPR-Cas9, which is a new genome editing technology that allows scientists to intervene with precision within the DNA.
“Cas9” stands for “CRISPR-associated protein 9,” an enzyme that can be used as a tool to remove copies of genes from the genome.
What Liu and team struggled with was getting Cas9 to only “snip off” the mutated copy of Tmc1 and prevent it from also disrupting the healthy copy. This struggle arose from the fact that the mutated and healthy copy differ in only one spot, making it more difficult for the enzyme to differentiate between the two.
The solution chosen by the researchers was to deliver Cas9, as well as the guide RNA that is used to direct it, encapsulated in a lipid-based compound. This is a method previously described by Liu and other investigators.
Lipid-encapsulated Cas9 is more efficient, as it can find its way to the targeted gene copy more easily and has a reduced chance of lingering for long enough to interfere with other DNA segments.
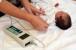
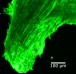
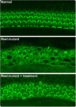
The researchers injected the lipid-encapsulated Cas9 and guide RNA complex into the cochlea of newborn mice with a faulty copy of Tmc1, with the effect that, after 8 weeks, the animals’ cochlear hair cells remained mostly intact.
By contrast, the animals that hadn’t been injected with the Cas9-guide RNA complex lost their cochlear hair to a great extent in that period.
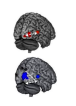
Using electrodes, Liu and colleagues proceeded to test the mice’s hearing capacity by monitoring brain activity in the regions associated with processing sound. They discovered that the animals that had not received the CRISPR-Cas9 intervention needed louder stimuli to react.
Around 4 weeks after the procedure, the mice that were injected with the Cas9 complex were able to perceive sounds that were 15 decibels lower than the ones needed to elicit a reaction from the control animals.
According to Liu, “That’s roughly the difference between a quiet conversation and a garbage disposal.”
Although this technique cannot yet be used to treat human cases, the researchers are hopeful that, in the future, this method will prevent total loss of hearing in many people exposed to hereditary risk factors for deafness.
Liu suggests that this treatment should be applied during childhood, to prevent the loss of cochlear hair as early as possible, since the damage, once done, is not usually reversible. “The conventional thinking in the field is that once you’ve lost your hair cells, it’s difficult to get them back,” he says.
Source: Medical News Today
Image credit: Wikimedia Commons

 29 Dec , 2017
Innovative Gene Editing Method May Prevent DeafnessFor the first time, researchers have used an innovative, state-of-the-art genome editing technique to prevent deafness in mice. There is hope that, in the future, they will be able to use this method to stop the loss of hearing in humans.According to data from the National Institute on Deafness and Other Communication Disorders, around two to three children in every 1,000 in the United States are born with a hearing impairment in one or both ears, and about 15 percent of adults have hearing problems.Moreover, the Centers for Disease Control and Prevention (CDC) note that 50 to 60 percent of hearing loss cases in babies are due to genetic factors, caused by the mutation of genes that “program” hearing.Recently, scientists have been experimenting with genome editing methods in the hope that they would be able to manipulate it so as to prevent the onset of total deafness due to genetic factors.Researchers at the Howard Hughes Medical Institute in Chevy Chase, MD, have now used precise genome editing technology called CRISPR-Cas9 on a mouse model to remove a gene variant that can lead to total loss of hearing.“We hope that the work will one day inform the development of a cure for certain forms of genetic deafness in people,” says David Liu, one of the researchers involved with the study.Liu and colleagues detail the process and their findings in a paper published in the journal Nature.One gene that has been associated with hearing is Tmc1. Mutations in this gene have been known to cause deafness, since they trigger the loss of hair cells in the cochlea, a part of the inner ear. Cochlear hair cells play an important role in hearing; they pick up vibrations and communicate with brain cells, thereby allowing the sensation to be processed. Mutated Tmc1 causes gradual hearing loss in both humans and mice, which allowed Liu and team to use the mouse model in their research.The scientists hypothesized that, if they could remove the mutated copy of the gene, they would be able to prevent total loss of hearing in the animals.Working with young mice, the researchers used CRISPR-Cas9, which is a new genome editing technology that allows scientists to intervene with precision within the DNA.“Cas9” stands for “CRISPR-associated protein 9,” an enzyme that can be used as a tool to remove copies of genes from the genome.What Liu and team struggled with was getting Cas9 to only “snip off” the mutated copy of Tmc1 and prevent it from also disrupting the healthy copy. This struggle arose from the fact that the mutated and healthy copy differ in only one spot, making it more difficult for the enzyme to differentiate between the two.The solution chosen by the researchers was to deliver Cas9, as well as the guide RNA that is used to direct it, encapsulated in a lipid-based compound. This is a method previously described by Liu and other investigators.Lipid-encapsulated Cas9 is more efficient, as it can find its way to the targeted gene copy more easily and has a reduced chance of lingering for long enough to interfere with other DNA segments.The researchers injected the lipid-encapsulated Cas9 and guide RNA complex into the cochlea of newborn mice with a faulty copy of Tmc1, with the effect that, after 8 weeks, the animals’ cochlear hair cells remained mostly intact.By contrast, the animals that hadn’t been injected with the Cas9-guide RNA complex lost their cochlear hair to a great extent in that period.Using electrodes, Liu and colleagues proceeded to test the mice’s hearing capacity by monitoring brain activity in the regions associated with processing sound. They discovered that the animals that had not received the CRISPR-Cas9 intervention needed louder stimuli to react.Around 4 weeks after the procedure, the mice that were injected with the Cas9 complex were able to perceive sounds that were 15 decibels lower than the ones needed to elicit a reaction from the control animals.According to Liu, “That’s roughly the difference between a quiet conversation and a garbage disposal.”Although this technique cannot yet be used to treat human cases, the researchers are hopeful that, in the future, this method will prevent total loss of hearing in many people exposed to hereditary risk factors for deafness.Liu suggests that this treatment should be applied during childhood, to prevent the loss of cochlear hair as early as possible, since the damage, once done, is not usually reversible. “The conventional thinking in the field is that once you’ve lost your hair cells, it’s difficult to get them back,” he says.Source: Medical News TodayImage credit: Wikimedia Commons
29 Dec , 2017
Innovative Gene Editing Method May Prevent DeafnessFor the first time, researchers have used an innovative, state-of-the-art genome editing technique to prevent deafness in mice. There is hope that, in the future, they will be able to use this method to stop the loss of hearing in humans.According to data from the National Institute on Deafness and Other Communication Disorders, around two to three children in every 1,000 in the United States are born with a hearing impairment in one or both ears, and about 15 percent of adults have hearing problems.Moreover, the Centers for Disease Control and Prevention (CDC) note that 50 to 60 percent of hearing loss cases in babies are due to genetic factors, caused by the mutation of genes that “program” hearing.Recently, scientists have been experimenting with genome editing methods in the hope that they would be able to manipulate it so as to prevent the onset of total deafness due to genetic factors.Researchers at the Howard Hughes Medical Institute in Chevy Chase, MD, have now used precise genome editing technology called CRISPR-Cas9 on a mouse model to remove a gene variant that can lead to total loss of hearing.“We hope that the work will one day inform the development of a cure for certain forms of genetic deafness in people,” says David Liu, one of the researchers involved with the study.Liu and colleagues detail the process and their findings in a paper published in the journal Nature.One gene that has been associated with hearing is Tmc1. Mutations in this gene have been known to cause deafness, since they trigger the loss of hair cells in the cochlea, a part of the inner ear. Cochlear hair cells play an important role in hearing; they pick up vibrations and communicate with brain cells, thereby allowing the sensation to be processed. Mutated Tmc1 causes gradual hearing loss in both humans and mice, which allowed Liu and team to use the mouse model in their research.The scientists hypothesized that, if they could remove the mutated copy of the gene, they would be able to prevent total loss of hearing in the animals.Working with young mice, the researchers used CRISPR-Cas9, which is a new genome editing technology that allows scientists to intervene with precision within the DNA.“Cas9” stands for “CRISPR-associated protein 9,” an enzyme that can be used as a tool to remove copies of genes from the genome.What Liu and team struggled with was getting Cas9 to only “snip off” the mutated copy of Tmc1 and prevent it from also disrupting the healthy copy. This struggle arose from the fact that the mutated and healthy copy differ in only one spot, making it more difficult for the enzyme to differentiate between the two.The solution chosen by the researchers was to deliver Cas9, as well as the guide RNA that is used to direct it, encapsulated in a lipid-based compound. This is a method previously described by Liu and other investigators.Lipid-encapsulated Cas9 is more efficient, as it can find its way to the targeted gene copy more easily and has a reduced chance of lingering for long enough to interfere with other DNA segments.The researchers injected the lipid-encapsulated Cas9 and guide RNA complex into the cochlea of newborn mice with a faulty copy of Tmc1, with the effect that, after 8 weeks, the animals’ cochlear hair cells remained mostly intact.By contrast, the animals that hadn’t been injected with the Cas9-guide RNA complex lost their cochlear hair to a great extent in that period.Using electrodes, Liu and colleagues proceeded to test the mice’s hearing capacity by monitoring brain activity in the regions associated with processing sound. They discovered that the animals that had not received the CRISPR-Cas9 intervention needed louder stimuli to react.Around 4 weeks after the procedure, the mice that were injected with the Cas9 complex were able to perceive sounds that were 15 decibels lower than the ones needed to elicit a reaction from the control animals.According to Liu, “That’s roughly the difference between a quiet conversation and a garbage disposal.”Although this technique cannot yet be used to treat human cases, the researchers are hopeful that, in the future, this method will prevent total loss of hearing in many people exposed to hereditary risk factors for deafness.Liu suggests that this treatment should be applied during childhood, to prevent the loss of cochlear hair as early as possible, since the damage, once done, is not usually reversible. “The conventional thinking in the field is that once you’ve lost your hair cells, it’s difficult to get them back,” he says.Source: Medical News TodayImage credit: Wikimedia Commons