Blog
-
FDA Approves Cochlear Implant for MRI with Internal Magnet in Place
 18 Jul , 2016
(The time spent without hearing is only for the duration of the MRI scan, as opposed to days waiting for a surgical incision to heal)
18 Jul , 2016
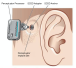
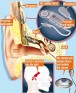
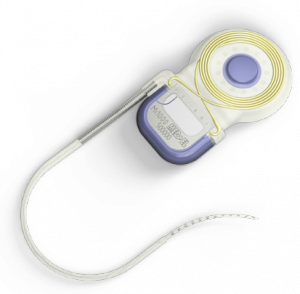
(The time spent without hearing is only for the duration of the MRI scan, as opposed to days waiting for a surgical incision to heal)Hearing implant manufacturer MED-EL has announced the FDA approval of its COMBI 40+ cochlear implant as conditionally safe with 1.5T MRI with the internal magnet in place. The COMBI 40+ first received FDA approval in August 2001, and was the first MED-EL cochlear implant offered in the United States.
Magnetic Resonance Imaging (MRI) machines are available in a range of strengths, called Tesla (T). The current standard strength is 1.5T in the United States, and the vast majority of MRIs performed are done with 1.5T machines.
MED-EL has MED-EL has been pursuing MRI compatibility for its implant recipients for some time. All MED-EL cochlear implants have been FDA approved for 0.2T scans without surgical removal of the magnet since 2001. In June 2013, the company was granted FDA approval for PULSAR, SONATA and CONCERT cochlear implants as conditionally safe for 1.5T MRI with the magnet in place. In January 2015, MED-EL announced the FDA approval of the SYNCHRONY cochlear implant, the first implant conditionally approved for high-resolution 3.0T MRI with the magnet in place–considered a breakthrough in MRI and cochlear implant technology.
“From the very beginning, MED-EL has engineered our cochlear implants to be future-ready so that our recipients can access the latest technology as it becomes available,” said Raymond Gamble, CEO and president of MED-EL North America. “As a company, we continuously strive to support our recipients through the life of their implant. This FDA approval demonstrates an unprecedented level of support in the cochlear implant industry. There is simply no other company that goes to this length to ensure that their end users can access technological advancements, including MRI.”
MRI capability is an important issue for people who have cochlear implants. People who live with chronic conditions, such as heart disease, stroke, cancer, and neuromas may require the regular use of MRI technology. Diagnostics with MRI were not routine at the time of the COMBI 40+ approval. However, usage has increased at a rate of 10% per year since 1996. In 2015, an estimated 37.8 million MR procedures were performed in approximately 8,465 sites in the United States.
According to MED-EL, people with other brands of cochlear implants must have surgery to remove the internal magnet before undergoing an MRI scan, and a second surgery is required to replace the magnet after the scan has been obtained. Even surgeries described as “minimally invasive” can cause pain and discomfort, come with an increased risk of surgical side effects such as scarring and infection, and add unnecessary cost from the surgery (including a new, sterile magnet) as well as lost wages during recovery.
Because no additional surgery is necessary for any of MED-EL’s implant users to receive an MRI, there is also no down time, aside from during the MRI, when the recipient needs to go without their audio processor, which must be removed for MRI scanning. Therefore, the time spent without hearing is only for the duration of the MRI scan, as opposed to days waiting for a surgical incision to heal. Medical professionals can download the latest MED-EL MRI safety guidance from the MED-EL website.
Austria-based MED-EL Medical Electronics is a provider of hearing implant systems with 29 subsidiaries worldwide. The family-owned business is considered one of the pioneers in the industry, founded by Austrian scientists Ingeborg and Erwin Hochmair, who developed the first microelectronic multi-channel cochlear implant in 1977. The cochlear implant was and remains the first replacement of a human sense, the sense of hearing. In 1990, the Hochmairs laid the foundation for the successful growth of the company when they hired their first employees. To date, the company has grown to more than 1,500 employees around the world. Today MED-EL a wide range of implantable solutions worldwide to treat various degrees of hearing loss, including cochlear and middle ear implant systems. People in over 100 countries enjoy the gift of hearing with the help of a product from MED-EL.
Source: MED-ELImage credit: MED-EL