Blog
-
Hearing the Light, “Optogenetics” Provides Bright New Prospects for Hearing Restoration.
 24 Jul , 2018
24 Jul , 2018
Hearing the Light, “Optogenetics” Provides Bright New Prospects for Hearing Restoration.
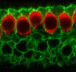
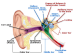
A group of German researchers have used light to make deaf gerbils hear using a method known as optogenetic stimulation, to create a new and improved cochlear implant. Based on their data, these light-based cochlear implants—called optical cochlear implants or oCI—may be the future for treating hearing impairment.
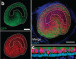
Using an improved rodent model, the group developed an oCI that uses light for precise stimulation of the auditory nerve in deaf adult gerbils. They characterized the light-induced activity in the auditory pathway using electrophysiological and behavioral analysis.
When asked what was unique about their study, Tobias Moser, M.D., at the University Medical Center Göttingen Institute for Auditory Neuroscience and InnerEarLab in Germany, and senior author on the study, offers that “we [are] the first, to our knowledge, [to] transfer the optogenetic ‘light-switch’ to the cochlear neurons in adult animals that are not genetically accessible.” He adds that “we show for the first time a behavior driven by cochlear optogenetics. As this behavior could be transferred to acoustic stimulation, we consider the optically evoked percept an auditory one, somewhat comparable to the acoustic one, i.e., hearing the light.”
The study entitled “Optogenetic stimulation of cochlear neurons activates the auditory pathway and restores auditory-driven behavior in deaf adult gerbils,” was published recently in Science Translational Medicine.
Optogenetics is a recently developed technology, pioneered by Ed Boyden, Ph.D., and Feng Zhang, Ph.D., during their graduate work in the Deisseroth Lab at Stanford University, that is used to control the activity of genetically defined neurons with light.
The demonstration of optogenetic stimulation of the auditory pathway was published four years ago, also by Dr. Moser’s group. In it, adeno-associated virus (AAV)-mediated gene transfer to mouse spiral ganglion neurons (SGNs) demonstrated activation of the auditory pathway. In their more recent paper, Dr. Moser’s group improves upon their previous system by using an animal model that more closely resembles the human system, the Mongolian gerbil. In comparison to mice and rats, this gerbil has a larger cochlea and its hearing extends to lower frequencies that are also used by the human ear. To test whether the electrophysiological responses triggered by optical stimulation had functional effects on behavior, the researchers used a behavioral assay (the shuttlebox paradigm of negative reinforcement) that first linked a sound and a behavior, then light and that behavior.
A shuttlebox consists of a box divided into two compartments separated by a hurdle over which the gerbils can jump (“shuttling” from one compartment to the other). Foot shocks are delivered through the floor of one side of the box, initiating the gerbils’ desire to jump to the other “safe” side. In this study, the shocks were given in coordination with acoustic clicks to train the gerbils. In time, they leapt over the hurdle whenever they heard the alarm.
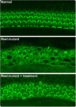
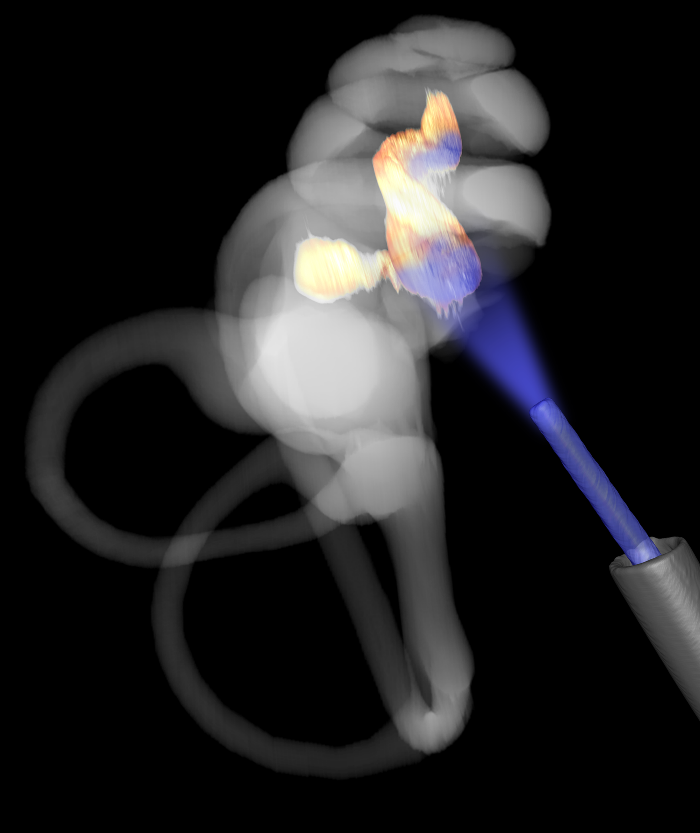
Next, the SGNs in the cochlea of the adult gerbils were injected with an AAV carrying the gene encoding the light-sensitive calcium translocating channelrhodopsin (CatCh). Light stimulation of the SGNs activated the neuron in the cochlea. Finally, the researchers implanted optical fibers to give off light within the cochlea. When the fibers were turned on to give off blue light, in the absence of any sound, the gerbils still jumped over the hurdle.
Lastly, the researchers restored hearing in a gerbil model of ototoxic deafness. After the gerbils’ cochlea were injected with kanamycin to kill hair cells and induce deafness, the oCI system was implanted. As in the previous experiment, the gerbils crossed to the safe compartment in the shuttlebox upon optogenetic stimulation, indicating that the oCI stimulation of SGNs restored activation of the auditory pathway in the deafened gerbils.
Rachael Richardson, Ph.D., senior research fellow at the Bionics Institute in Melbourne, Australia, tells GEN that, “the primary significance of this work is the demonstration and achievement of some of the major goals needed to bring oCI to the next step.” She adds, “perhaps the biggest leap made in this paper was putting the opsins into the SGNs” because “the standard gene-therapy methods don’t work well in these cells.” She also cites the behavioral data in the paper as an impressive proof of concept.
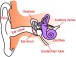
About 360 million people suffer from disabling hearing impairment (HI). The most common form, sensorineural HI, results from cochlear dysfunction or degeneration typically involving loss of sensory hair cells. The current generation of cochlear implants, worn by more than 324,000 people across the world, are electronic devices that can provide a sense of sound to a person who is profoundly deaf or severely hard-of-hearing. These electrical cochlear implants (eCI) directly stimulate the auditory nerve to the brain by bypassing the damaged portion of the ear.
A cochlear implant is not a hearing aid and does not restore normal hearing. What it does is help someone understand speech. However, users can have a tough time comprehending speech in environments with a lot of interfering noise—which is one of the areas where the improved restorative capabilities of an oCI would be a significant improvement over the current model.
A recent review by Dr. Richardson states that, “optogenetic-based approaches hold the greatest potential and viability amongst optical techniques for application in the cochlea. The future success of this approach will be governed by advances in the targeted delivery of opsins to auditory neurons, improvements in channel kinetics, development of optical arrays, and innovation of opsins that activate within the optimal near-infrared therapeutic window.”
When asked if oCIs will be made available to patients, Dr. Richardson says that “there are still challenges to overcome before that can happen, many of which have to do with the long-term nature of oCIs.” For example, she says that the CatCh opsins and the optical implant have to be proven safe for use over long periods of time. This will, most likely, take close collaboration with material scientists and engineers to ensure that the implant is small enough, the microLEDs face the right way, and it is hermetically sealed. Despite these challenges, Dr. Richardson is optimistic and says that making oCIs available to the HI community is an achievable goal. Dr. Moser hopes that “this will happen in the 2020s.”
Dr. Richardson adds that “this field has never been more exciting” because there are many treatment options that could be just around the corner. Up until now, the options have been limited to hearing aids or eCIs. “The innovative strategies done in this paper are the key component to advancements in the field.” Taken together, the field is opening up choices for people with hearing loss that are simply not there right now.
Source: Julianna LeMieux, Ph.D., Genetic Engineering & Biotechnology News
Image credit: University Medical Center Göttingen